|
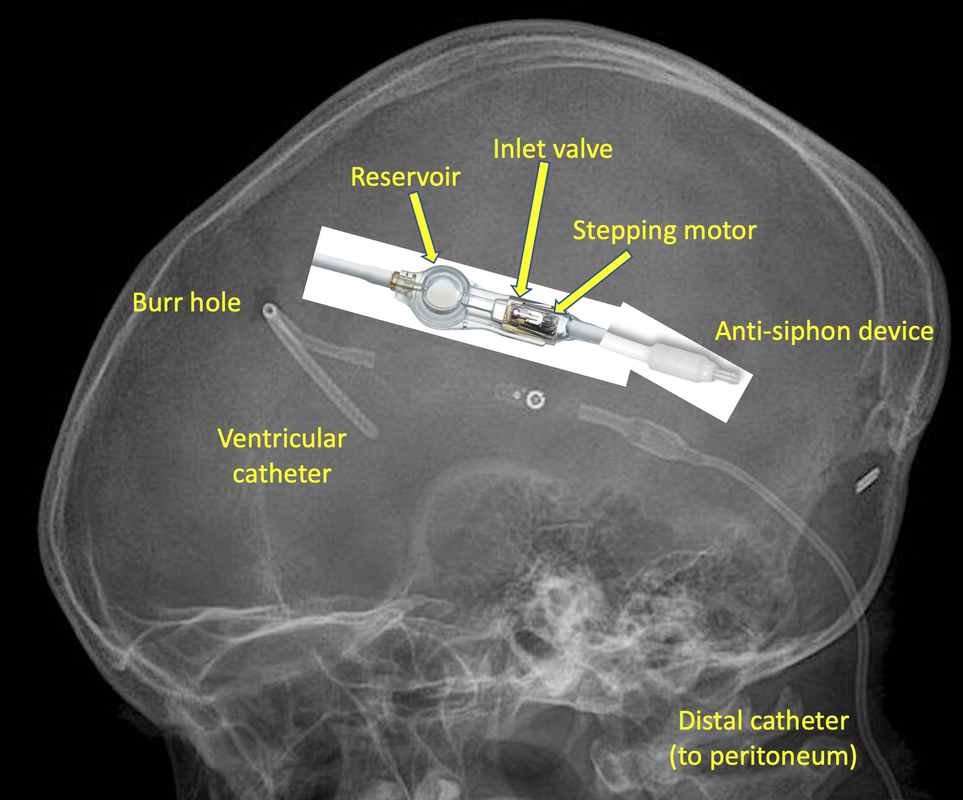
Cerebrospinal fluid (CSF) shunts, used primarily for treatment of hydrocephalus, are among the most commonly implanted neurosurgical devices. The typical shunt consists of 3 components — (1) a silastic catheter placed through a burr hole into a ventrical to suction out CSF, (2) a subcutaneous one-way valve mechanism for directing flow, and 3) a distal catheter to transport CSF to a distal absorption site. The distal site is typically the peritoneal cavity, but can be the pleural space, heart (right atrium), or central venous system.
|
In addition to these basic components, shunt systems may also incorporate a subcutaneous reservoir or “antechamber” with a silicone elastomer dome that can be manually pumped to test for shunt patency or used for needle aspiration/injection. Many systems also include an anti-siphon or "anti-gravity" device to reduce excess flow of CSF when the patient assumes the upright posture.
About a half dozen companies manufacture shunt tubing, valves, and accessories world-wide, including: AESCULAP-MIETHKE® (m.blue™, ProGAV®, proSA, SHUNTASSISTANT®), Integra (CODMAN-HAKIM®, CODMAN® CERTAS™, OSV II®); Medtronic (PS Medical, Strata II); Natus® (Natus® DP, Novus™, Contour-Flex™, Ultra VS™, Pudenz); and SOPHYSA (Polaris®, Sophy® Mini, Siphonx®)
The shunt valve is the critical component of the system with many variations and designs available. The simplest type is a fixed pressure valve that opens only when a single predetermined CSF pressure differential is exceeded. These valves may operate via deflection of a silicone membrane (diaphragm) and be incorporated into the base of a reservoir. A second common design is a movable ball held in place by a compression spring, whose elasticity determines the valve's opening pressure. The major disadvantage of fixed pressure valves is that a single specific pressure must be chosen before surgery.
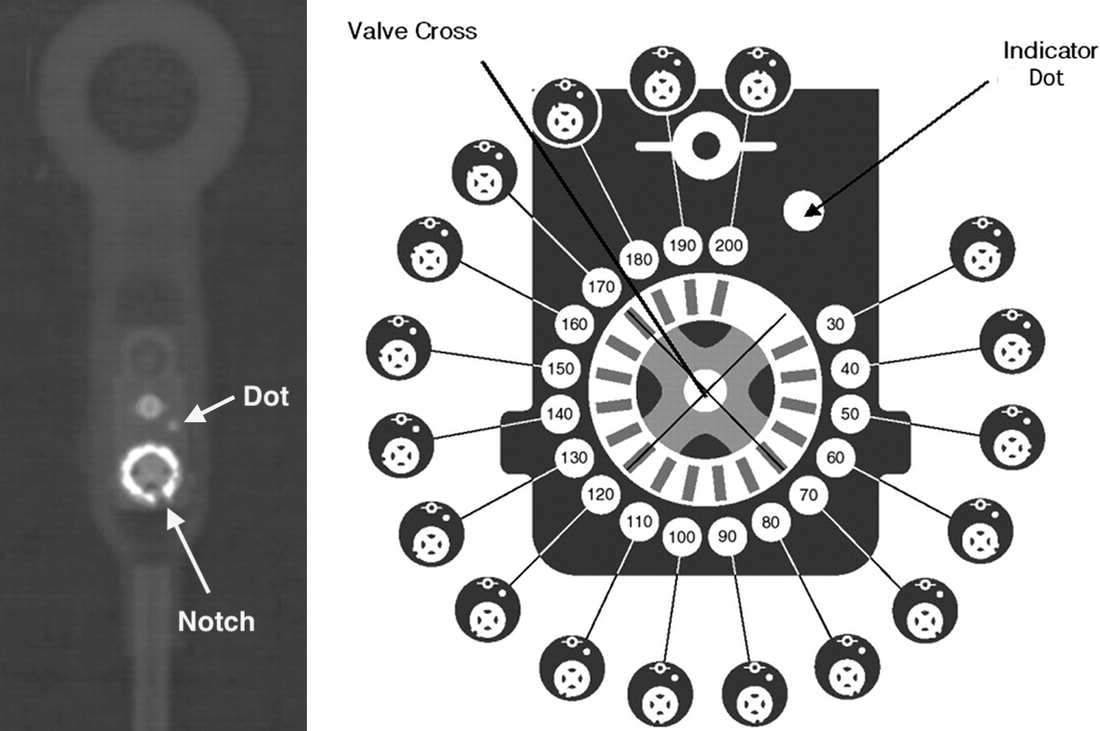
Programmable (adjustable) valves allow opening pressures to be changed non-invasively. In addition to a ball and spring inlet valve, a separate magnetically-activated mechanism allows additional incremental pressure regulation in typical increments of 10-20 mm H2O. The design of the programmable component varies by manufacturer. The Integra Codman-Hakim® Valve uses a "stepping motor" − a rotating disk embedded with 10 permanent micro-magnets that can be raised or lowered along a spiral plastic “staircase” using an external magnetic programmer. The Sophysa Polaris® system operates through a modified spring/ball mechanism whose tension is controlled by the position of a rotating bar containing two small magnets. After programming, the pressure set for of each valve can be determined by a magnetic compass or the position of radio-opaque markers on x-ray (dependent on the model).
Several other related drainage devices should be mentioned here:
- Extraventricular drains (EVDs) are catheters that divert CSF from the ventricle to a sterile container outside the patient. The line may or may not contain an anti-reflux valve. EVDs are used for temporary relief of acute hydrocephalus or increased intracranial pressure when long-term therapy with a shunt is not anticipated. The CSF collection container is raised or lowered on an IV pole to control/measure intracranial pressure and CSF flow. If a patient with an EVD is brought into the MRI suite for scanning, make sure the IV pole is replaced by an MR-compatible one! Also, some systems have small leveling lasers that should also be removed.
- Lumbar shunts and drains are similar to ventricular shunts and EVDs except they siphon CSF from the spinal canal (into the peritoneal cavity or into an external system respectively).
- Non-shunt reservoir systems consist of only a ventricular catheter and reservoir, often manufactured as a single piece. No shunt valve or distal components are present. These systems are primarily used to deliver intraventricular chemotherapy for cancer or infection and to allow intermittent sampling of CSF. Commonly used models include Rickham®, Ommaya®, and Holter® reservoirs. Depending on their composition, they are either MR Safe or MR Conditional.
- Subdural Evacuating Port System (SEPS)™ is a device made by Medtronic used for the treatment of chronic subdural hematomas. A bolt-like evacuating port made of stainless steel is screwed into a skull burr hole that communicates with the subdural space while the other end is connected to silicone tubing and a plastic suction bulb. The manufacturer has declared SEPS to be MR Unsafe (but it is unclear to me how the risk of such a device is greater than that of standard bolts, most of which are MR Conditional.)
MRI Safety Considerations
Many fixed pressure valve shunt systems and reservoirs are entirely made of non-metallic components and are thus MR Safe. The shunt tubing is made radio-opaque by embedded barium, so is also safe at all field strengths. Shunt connectors are typically made of titanium, making them (trivially) MR Conditional. Fixed valves containing springs are all rated MR Conditional up to 3.0T, but the springs are typically made of 316 nonferromagnetic stainless steel that produce only a small artifact.
Programmable valves all contain small magnets in addition to springs and other components made of 316 stainless steel, titanium, or tantalum. These will definitely produce moderately large susceptibility artifacts and potentially be affected by the magnetic field. All currently produced programmable shunt systems including anti-siphon devices are considered MR Conditional up to 3.0T. In addition to exposure to MRI fields, patients are warned that devices such as cellphones and headphones be kept at least 5 cm (2") from the valve mechanism.
The main risk of MRI is unintentional resetting of the valve pressure. This occurs in up to 40% of patients with Codman-Hakim® valves and even up to 10% of valves marketed as "MR immune". Within 4 hours after exposure to MRI, all patients must have their shunt pressures interrogated and potentially reprogrammed to the original settings.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Abbott R, Kobets A, Burns JG. The ISPN Shunt Guide. The ISPN Guide to Pediatric Neurosurgery. 2021. [Available from this link]
Capitanio JF, Venier A, Mazzeo LA, et al. Prospective study to evaluate rate and frequency of perturbations of implanted programmable Hakim Codman valve after 1.5-Tesla magnetic resonance imaging. World Neurosurg 2016; 88:297-299. [DOI LINK]
Inoue T, Kuzu Y, Ogasawara K, Ogawa A. The effect of 3.0-tesla magnetic resonance imaging on various pressure-programmable shunt valves. European Neurological Review 2006; 6:1-3. [DOI LINK]
Kraus M. Radiologic identification of VP-Shunt valves and adjustment. Kinderneurochiurgie Leipzig. 2019. [Available from this link]
Lollis SS, Mamourian AC, Vaccaro TJ, Duhaime A-C. Programmable CSF shunt valves: radiographic identification and interpretation. AJNR Am J Neuroradiol 2010; 31:1343-46. [DOI LINK]
Mirzayan MJ, Klinge PM, Samir M, et al. MRI safety of a programmable shunt assistant at 3 and 7 Tesla. Br J Neurosurg 2012; 26:397-400. [DOI LINK] (OK at 3T but not 7T)
Moghtader D, Crawack H-J, Miethke C, et al. Assessment of MRI issues for a new cerebral spinal fluid shunt, gravitational valve (GV). Magn Reson Imaging 2017; 44:8-14. [DOI LINK]
Soler GJ, Bao M, Jaiswal D, et al. A review of cerebral shunts, current technologies, and future endeavors. Yale J Biol Med 2018; 91:313-321.
Tandon V. Shunt Technologies. On-line presentation (2017) downloaded from this link May 2021. (lots of pictures and diagrams of the internal workings of various shunts and accessory equipment)
Abbott R, Kobets A, Burns JG. The ISPN Shunt Guide. The ISPN Guide to Pediatric Neurosurgery. 2021. [Available from this link]
Capitanio JF, Venier A, Mazzeo LA, et al. Prospective study to evaluate rate and frequency of perturbations of implanted programmable Hakim Codman valve after 1.5-Tesla magnetic resonance imaging. World Neurosurg 2016; 88:297-299. [DOI LINK]
Inoue T, Kuzu Y, Ogasawara K, Ogawa A. The effect of 3.0-tesla magnetic resonance imaging on various pressure-programmable shunt valves. European Neurological Review 2006; 6:1-3. [DOI LINK]
Kraus M. Radiologic identification of VP-Shunt valves and adjustment. Kinderneurochiurgie Leipzig. 2019. [Available from this link]
Lollis SS, Mamourian AC, Vaccaro TJ, Duhaime A-C. Programmable CSF shunt valves: radiographic identification and interpretation. AJNR Am J Neuroradiol 2010; 31:1343-46. [DOI LINK]
Mirzayan MJ, Klinge PM, Samir M, et al. MRI safety of a programmable shunt assistant at 3 and 7 Tesla. Br J Neurosurg 2012; 26:397-400. [DOI LINK] (OK at 3T but not 7T)
Moghtader D, Crawack H-J, Miethke C, et al. Assessment of MRI issues for a new cerebral spinal fluid shunt, gravitational valve (GV). Magn Reson Imaging 2017; 44:8-14. [DOI LINK]
Soler GJ, Bao M, Jaiswal D, et al. A review of cerebral shunts, current technologies, and future endeavors. Yale J Biol Med 2018; 91:313-321.
Tandon V. Shunt Technologies. On-line presentation (2017) downloaded from this link May 2021. (lots of pictures and diagrams of the internal workings of various shunts and accessory equipment)
Related Questions
What does it mean when an implant or device is labeled "conditional"?
Can patients with intracranial pressure monitors be scanned?
What does it mean when an implant or device is labeled "conditional"?
Can patients with intracranial pressure monitors be scanned?